Radioactive Displacement Law
Soddy and Fajan proposed displacement laws concerning the daughter nucleus produced during an alpha and beta decay. We state them below:
It emits the alpha particle when the daughter nucleus is formed. The atomic number is less by 2 units and the mass number is less by 4 units than the parent nucleus.
The beta particle is emitted, when the daughter nucleus is formed, where the mass number is the same as the parent nucleus and the atomic number is more by 1 unit than the parent nucleus.
Kindly note that in a Nuclear reaction, the element formed by radioactivity identified a change in atomic number, not by the mass number.
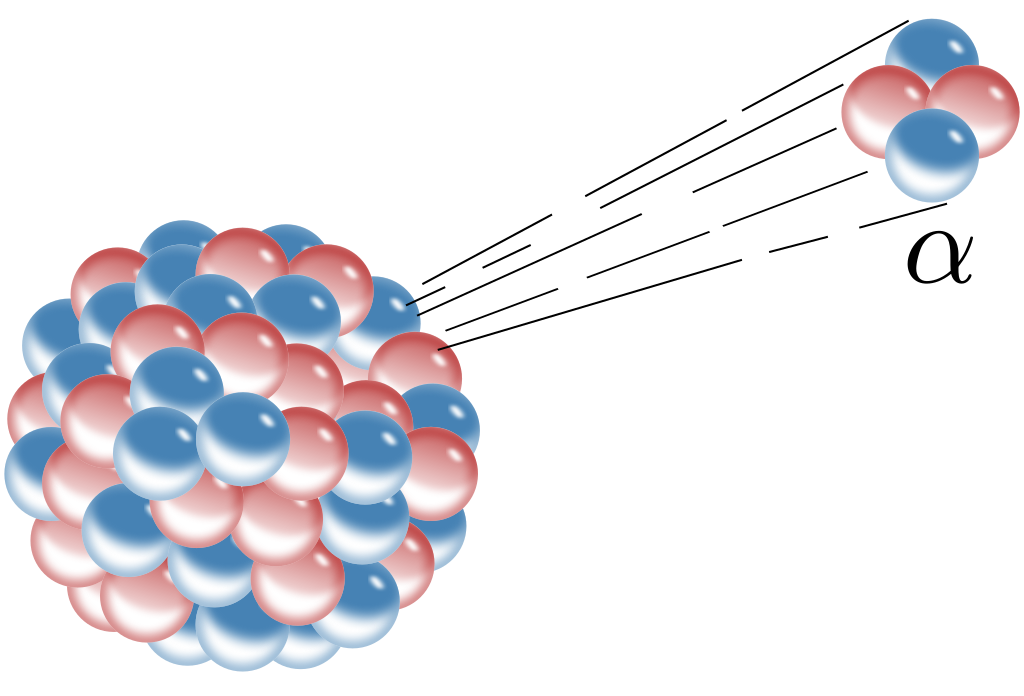
Alpha Decay
Radioactivity in which an unstable parent nucleus emits an alpha particle by daughter nucleus formation is called ‘Alpha Decay’.
Example: Decay of Uranium (U238 ) to thorium ( Th234) with the emission of an alpha particle.
92 U 238 à90 Th 234 + 2 He 4
From the above, we see, in alpha decay, mass number decrease by 4 and atomic number decreases by two.
Beta Decay
Radioactivity in which an unstable parent nucleus emits a Beta particle by daughter nucleus formation is called ‘Beta Decay’.
Example: Beta decay of Phosphorus.
15 P 32 à16 S 32 + -1 e 0 (Beta Decay)
In the beta decay, there is no change in the mass number but there is a change in atomic, the atomic number increased by one.
Gamma Decay
In gamma decay, the energy level of the nucleus only changes. The atomic number and mass number remain the same.